The chemicals sector faces a £2bn hit of post-Brexit red tape, twice the cost of initial industry estimates, as Britain sets up its own regulatory regime, ministers have warned.
If you sell chemicals, there is an opportunity to potentially reduce registration costs.
The BRC are responding to the consultation, but would like to hear your views by Monday 15th August.
The UK REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) Regulation is one of the main pieces of legislation for the regulation of chemicals in Great Britain. It established the UK REACH regime (UK REACH), which regulates the use of substances in Great Britain as EU REACH continues to apply in Northern Ireland.
UK REACH requires substances that are manufactured in, or imported into, GB to be registered with the Agency for UK REACH (the Health and Safety Executive (HSE). Registrations include information on the hazards, uses and exposure of the substance. Registration information is used by HSE for regulatory purposes and by the registrants to identify appropriate risk management measures for themselves and other users down the supply chain.
In response to concerns raised by stakeholders around the cost of acquiring the data to complete their registrations, the government is working with stakeholders to explore an alternative transitional registration model. The aim of this model is to reduce costs to businesses of transitioning from EU REACH to UK REACH whilst maintaining or improving existing human health and environment protections, in line with our international commitments.
The first of the current registration submission deadlines is in October 2023, therefore amendments to the current legislation are necessary to extend the deadlines to ensure there is sufficient time for substantive development of the policy, and to make operational and legislative changes to implement the new model. Industry will also need time to prepare for compliance with it. Extending the deadlines will reduce the likelihood of companies making nugatory investments in complying with current deadlines and data requirements. It will allow them time to plan their business decisions in relation to the extended deadlines.
This consultation also covers the proposal to extend the legislative timelines for the UK regulator to carry out the compliance checks on 20% of registration dossiers required under Article 41 of the UK REACH Regulations. These need to be amended to ensure that they apply after the relevant submission dates have passed, otherwise, no data may have been submitted for the Agency to carry out compliance checks on.
Proposed options:
The BRC would like to know your preferred option. Let me know by 15th August.
Baseline (Do Nothing) – do not change the current submission deadlines (27 October 2023, 27 October 2025, and 27 October 2027). Defra is currently working with industry, NGOs and key stakeholders to develop an alternative transitional registration model which looks to reduce the burdens associated with the submission of information, while improving the understanding by industry and regulators of the risks connected with the use of, and exposure to, chemicals in Great Britain.
There are impending deadlines for the submission of information under the current legislation to which industry are legally bound. Moving to an alternative registration model is likely to change the information industry would have to provide. With a ‘do nothing option’, the first deadline of 27 October 2023 will fall before the government has had time to develop and legislate for the alternative model. This will cause considerable uncertainty about what companies’ duties are and what steps they should be taking to meet them. There is also a risk that industry could start making a nugatory investment towards acquiring data that may not be necessary under the criteria set out in the alternative transitional registration model being developed.
Option 1 - Move the submission deadlines for each tonnage band back by 3 years. Option 1 would move the current submission deadlines back by 3 years as illustrated in Table 2 below.
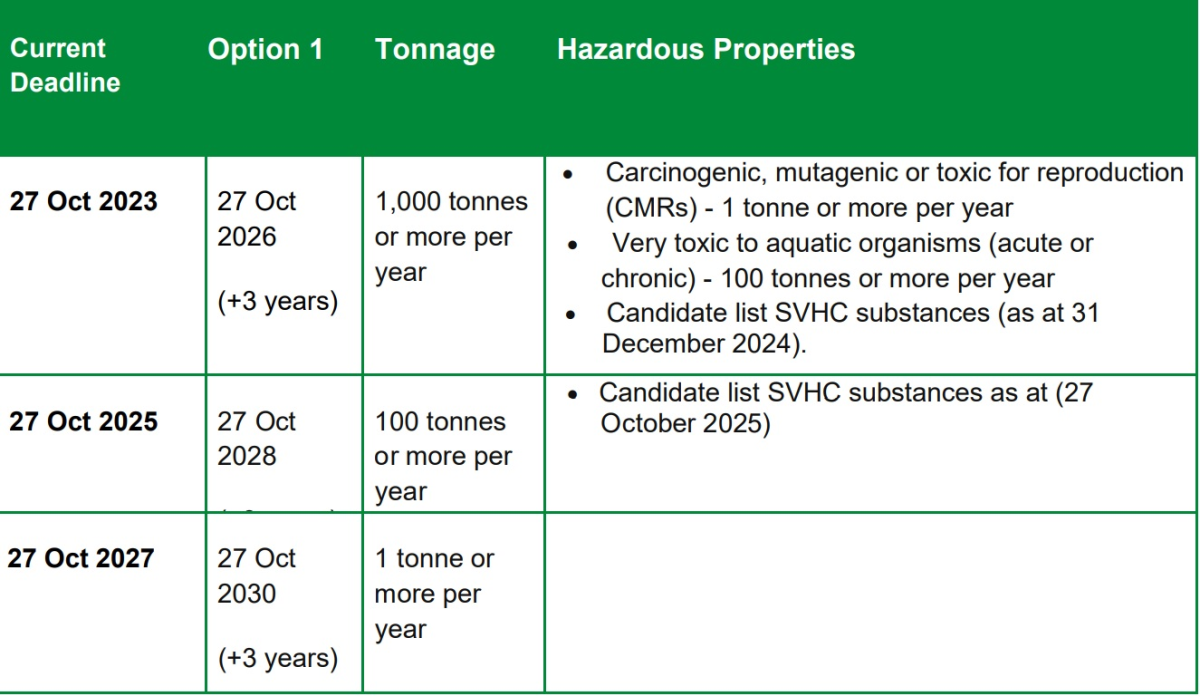
This should give the government time to introduce the alternative model and those subject to the first deadline time to prepare to comply with it. It would also give those subject to subsequent deadlines the same amount of time they have now to take account of what is done by those subject to earlier deadlines. As set out in the Article 1 Consistency Statement, the government considers that this option would be consistent with Article 1 of the UK REACH Regulation. In particular, with the purpose of ensuring a high level of protection for human health and the environment and the free circulation of substances.
Option 2 - Move the first submission deadline by 3 years to October 2026, the second by 2 years to October 2027, and the third by 1 year to October 2028.
Option 2 is the preferred option. Option 2 would move the first submission deadline back by 3 years, the second by 2 years and the final deadline by just one year as illustrated in Table 3 below.
Developing a new model is highly technical and complex and time is needed to develop a firm proposal. If a suitable model is found, operational (e.g., IT development) and legislative changes would need to be made to implement it.
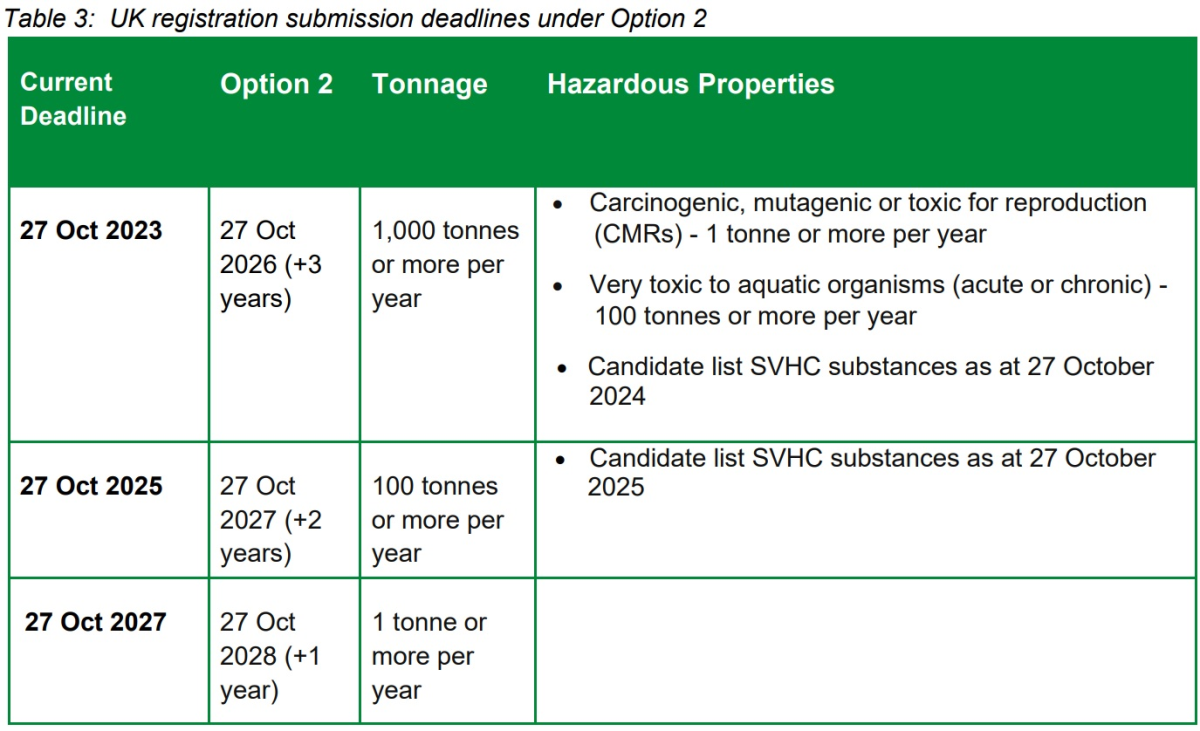
Moving the first submission deadline back by 3 years should give the government time to introduce the alternative transitional registration model and those subject to that deadline time to comply with it. Option 2 has the advantage of the transitional registration data being received by HSE earlier than under Option 1. Under this option, those subject to the second two submission deadlines would have less time to take account of what those subject to earlier submission deadlines did.
We do not consider that there would be any significant impacts to industry as a result of the reduced gaps between submission deadlines, and believe any disadvantages are outweighed by the benefits of HSE receiving the transitional registration data sooner. As set out in the Article 1 Consistency Statement, the government considers that this option would be consistent with Article 1 of the UK REACH Regulation. In particular, with the purpose of ensuring a high level of protection for human health and the environment and the free circulation of substances.
