Preparing for the new us cosmetic regulations: What UK and global businesses need to know
WHAT IS THE NEW MODERNIZATION OF COSMETICS REGULATION ACT?
Starting in 2023, MoCRA will introduce significant changes to U.S. cosmetics regulations, including a new concept of a U.S. “Responsible Person” (RP), new labelling requirements, mandatory product listing and facility registration, reporting of serious adverse events, and finished product safety assessment. The regulation will also establish a new Good Manufacturing Practice (GMP) standard for cosmetic products manufactured in the U.S.
Products that fail to comply with the new requirements will be prohibited. The FDA has been given additional enforcement authority where products may pose a significant risk to consumers. These include access to product information records, issuing mandatory recalls and suspension of facility registrations.
These regulatory updates have been in progress for over a decade, and many of the changes bring U.S. cosmetics regulation closer to that of U.S. over-the-counter drugs and to the EU Cosmetics Regulation, widely considered the industry “gold standard.”
Given the substantial implications of the new regulations, it is imperative for all global companies selling cosmetic products in the U.S. to begin preparing as soon as possible. The team of regulatory and safety experts at Exponent have compiled a summary of the key considerations and initial steps to take regarding these changes.
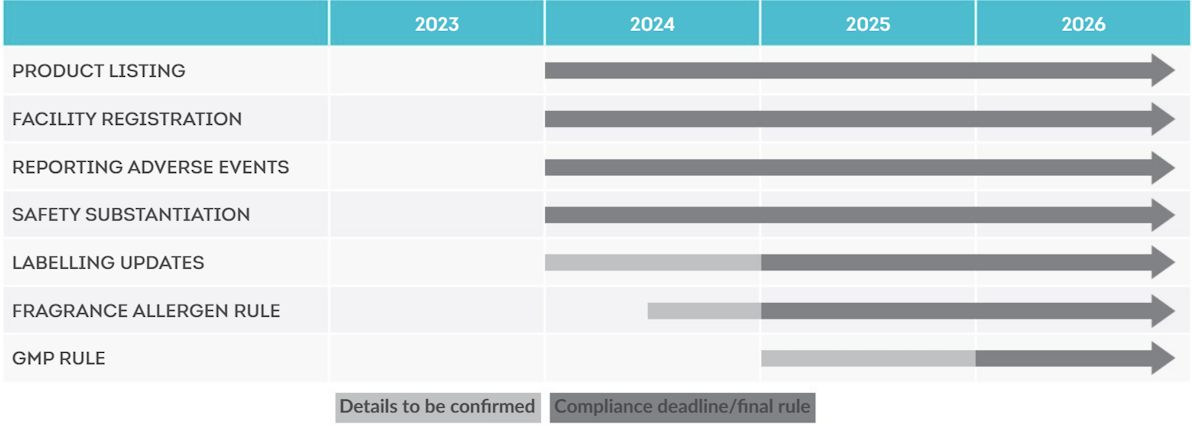
MOCRA IMPLEMENTATION TIMELINE
KEY PROVISIONS OF MoCRA
1. Mandatory Site Registration & Product Listing
By the end of December 2023, all facilities that manufacture or process cosmetic products distributed in the U.S. must register with FDA. Registration must include the facility name and contact information (international facilities will require a specified U.S. agent) and a list of the brands that are produced or processed at the facility.
As of March 2023, the FDA has ceased accepting submissions to the Voluntary Cosmetic Registration Program (VCRP) and will be implementing a new system for facility registrations and cosmetic product listings. The FDA has advised that additional information on this new system will be available in the coming months.
2. Current Good Manufacturing Practice (cGMP) Requirements
The FDA plans to introduce cosmetics GMP requirements in line with national and international standards in the next 2-3 years. Once enacted, any product processed or manufactured outside of the applicable cGMP standards will be deemed non-compliant.
3. Mandatory Reporting of Adverse Health Events
4. Safety Substantiation for Cosmetic Products
Effective starting December 29, 2023, the RP must maintain records supporting that each cosmetic product has “adequate substantiation of safety.” The term adequate substantiation of safety is defined in Section 608(c)(1) as “tests or studies, research, analyses, or other evidence or information that is considered, among experts qualified by scientific training and experience to evaluate the safety of cosmetic products and their ingredients, sufficient to support a reasonable certainty that a cosmetic product is safe.’’
Further clarity on the expected content and data requirement for a U.S. cosmetic safety assessment is expected this year. There may be a new requirement for testing beyond what is currently required by the EU and UK for a cosmetic product safety assessment.
5. Mandatory Product Labelling Information
By the end of 2024, all cosmetic products must be labelled with a U.S. domestic address, telephone number, or electronic contact information for the RP.
The FDA also plans to introduce mandatory labelling of fragrance allergens. While this is already a requirement for cosmetic products in the EU and UK, it is uncertain whether the FDA will adopt the same list of allergens and threshold levels as the EU. More information on this is expected during 2024.
WHAT PRACTICAL FIRST STEPS CAN COMPANIES TAKE NOW TO START PREPARING FOR MoCRA?
These new regulations will have significant implications for all UK and global companies selling cosmetic products in the U.S.
Exponent, along with other industry leaders, emphasises the importance of prioritising consumer safety, especially due to the high level of expertise needed to meet MoCRA requirements and the increased risk of non-compliance and resulting litigation. For many companies, this may require new employee hires with regulatory or toxicology expertise or outsourcing work to reputable external agencies for regulatory, toxicology, and testing services to ensure compliance. Exponent can help businesses review product ranges, identify potential compliance gaps, and get plans in place to comply with MoCRA.
To find out more about Exponent International and the services they provide to the retail industry, click here.
This article was also published in The Retailer, our quarterly online magazine providing thought-leading insights from BRC experts and Associate Members.


